Your Cart is Empty


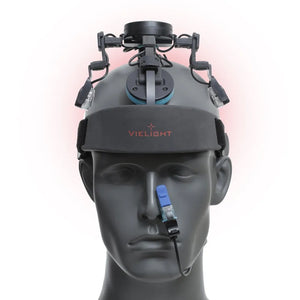



Vielight Neuro Duo 4
$2,399.00$0.00
- ✓ Questions? Call: 351 200 4050
- ✓ Sleep Recharged is a trusted Dealer
Neuro Duo 4 Transcranial Brain Photobiomodulation (tPBM) Device
As the world’s only transcranial-intranasal brain photobiomodulation (PBM) device, the Vielight Neuro Duo is is the result of years of engineering and research.
Clinical research with our technology has produced improvements in cognition, memory and blood flow.
The Vielight Neuro Duo comes with both Alpha and Gamma modes.
- Gamma (40Hz): focus, memory, brain energy.
- Alpha (10 Hz): relaxation and sleep improvement.
Vielight technology quality is guaranteed by medical grade certification.
Unlock Your Brain's Potential with the Vielight Neuro Duo
The Vielight Neuro Duo represents a powerful and extensively researched brain photobiomodulation (tPBM) device, featuring cutting-edge intranasal technology. It's designed to be safe, effective, and exceptionally user-friendly, offering the potential to unlock your brain's capabilities. Our patented transcranial-intranasal technology provides a unique and potent approach to stimulating the Default Mode Network in the brain.
The Neuro Duo emits pulsed near-infrared (NIR) energy through the cranium, initiating the process of photobiomodulation. Our engineering prowess allows us to extract one of the highest levels of power (~100 mW/cm2) from a near-infrared LED energy source, all while ensuring there is no heat generated.
This innovative device operates at two crucial frequencies:
- Gamma (40 Hz): Linked to enhanced focus, improved information processing, memory boost, and increased mental energy.
- Alpha (10 Hz): Supports mindfulness, facilitates learning, promotes relaxation, and can contribute to improved sleep.
Choose the Vielight Neuro Duo to embark on a journey of brain stimulation, where you can potentially experience amplified focus, cognitive performance, memory enhancement, relaxation, and better sleep. Our rigorous research and cutting-edge technology combine to offer you a powerful tool to tap into your brain's potential.
- Vielight technology quality is guaranteed by medical grade certification.
- Vielight technology is supported by a two-year manufacturer’s warranty from the time of delivery.

Why the nose?
Being just 3 inches from the brain, the intranasal channel is the most efficient channel for photobiomodulation of the ventral (underside) brain area. These ventral structures within the brain’s core have important functions.
Engineering behind the Vielight Neuro.
The Vielight Neuro enables direct contact with the scalp’s surface to maximize energy transmission and penetration while microchip-boosted cold LED diodes generate substantial power without releasing heat. Additionally, our patented intranasal technology enables photobiomodulation of the ventral brain areas.


Vielight Neuro – NIR light energy penetration.
Effects of the Vielight Neuro.
Default-mode network activity in
the PBM group—(A) baseline and (B) week
12 and in the usual care group—(C) baseline and (D) week 12. The posterior cingulate cortex (1, -61, and 38) was used as
seed in the analysis
Comparing EEG Power Spectrum:
Active vs Sham tPBM Using Non-Parametric Cluster-Based Permutation Test.


Non-parametric cluster-based permutation test comparing the rest EEG power spectrum between active and sham tPBM. Topographical maps are color-coded according to the permutation tests t-values. Clusters of electrodes with significant difference between the two conditions are marked in ‘+’ sign (p < 0.05 and αcluster = 0.01). (a) Difference between post and pre active tPBM. (b) Difference between post and pre sham tPBM. (c) Difference between pre active and sham tPMB. (d) Difference between post active and sham tPMB.
tPBM's Impact on Resting-State EEG:
Active vs Sham Stimulation Compared by Frequency Bands.
Influence of tPBM on resting-state electroencephalography. Box plot illustrates the median and range of power spectrum across all electrodes for each oscillatory frequency bands. (a) Effect of active tPBM on power spectrum pre (green line) and post (red line). (b) Effect of sham tPBM on power spectrum pre (green line) and post (red line). (c) Difference between Active and Sham tPBM: Change of power spectrum Post-Pre for active (red line) and sham (green line) tPBM. Active versus sham stimulation revealed significant lower alteration in delta and theta power and higher change in alpha, beta and gamma frequency bands.

Specifications
| Manufacturer |
Vielight Inc |
|---|---|
| LED Power |
1000 mW per module |
| Power Density | Irradiance |
100 mW/cm² (3) : Rear Transcranial LEDs |
| Est. Irradiance Area |
400cm² (+/- 50cm²) including Intranasal module footprint |
| Targeted Brain Network |
Default Mode Network |
| Head Size Suitability |
Minimum age: 5 years old based on published study data |
| Medical Grade Certification |
ISO13485 and MDSAP |
| Safety Certification |
TÜV Rheinland |
| Warranty |
24 months |
Clinical Research
| Condition | Topic | Institution | Vielight Device | Research Type | Status |
|---|---|---|---|---|
|
University of California San Francisco | US Veterans Affairs | Vielight Neuro Gamma | Clinical Study | Completed |
|
Harvard Medical School | Boston University School of Medicine | Vielight Neuro Gamma | Clinical Study | Completed |
|
Unity Health, Toronto | Vielight Neuro Gamma | Clinical Study | Analysis Stage |
|
University of Florida | University of Arizona | National Institute on Aging | Vielight 810 [MIP] | Phase 2 Clinical Trial (n=168) | Ongoing |
|
St Michael’s Hospital | Vielight Inc | Vielight Neuro Gamma | Phase 3 Clinical Trial (n=228) |
Ongoing |
|
University of California San Francisco | US Veterans Affairs | Vielight Neuro Gamma | Clinical Study | Results Analysis |
|
Department of Neurology, University of Utah | Vielight Neuro Gamma | Clinical Trial (n=49) | Pre-publication |
|
Boston University School of Medicine | Harvard Medical School | Vielight Neuro Gamma | Clinical Study | Completed |
|
VA Advanced Imaging Research Center, San Francisco VA Health Care System | Vielight Neuro Gamma | Clinical Study | Completed |
|
Boston Veterans Affairs Research Institute | Vielight Neuro Gamma | Clinical Study | Ongoing |
|
University of Sydney | Vielight Neuro Gamma | Clinical Study | Completed |
|
University of Florida | Vielight 810 [MIP] | Phase 2 Clinical trial (n=135) | Ongoing |
|
University of Sydney | University of Brisbane | Vielight Neuro Gamma | Clinical Study | Registered |
|
University of California San Francisco | US Veterans Affairs | Vielight Neuro Alpha | Clinical Study | Completed |
|
Neurodevelopment Division, Istituto di Neuroscienze, Italy | Vielight Neuro Duo | Clinical Study | Completed |
|
Vielight Inc | Vielight RX Plus | Pivotal Phase 3 Clinical Trial (n=295) | Completed |
|
University of Toronto | Vielight Inc | Vielight Neuro Gamma | Clinical Study | Completed |
|
Vielight Inc | Vielight Neuro Alpha | Clinical Study | Pre- Publication |
|
Princeton University | University of Alberta | Vielight Inc | Vielight Neuro Alpha | Research Study | Completed |
|
Vielight Inc | Vielight RX Plus | Pivotal Phase 3 Clinical Trial (n=295) | Completed |
|
Harvard Medical School | Vielight Blue [MIP] | Scientific Study | Completed |
|
Harvard Medical School | Vielight Blue [MIP] | Scientific Study | Completed |
|
Harvard Medical School | Vielight Blue [MIP] | Scientific Study | Completed |
|
University of Deusto, Spain | University of Montpellier, France | Pennsylvania State University | Vielight Neuro Gamma | Clinical Research (n=56) | Completed |
|
University of Alberta | Polytechnic University of Turin | Vielight Neuro Alpha | Scientific Study | Completed |
- Alzheimer’s Disease (Dementia)
Effects of Home Photobiomodulation Treatments on Cognitive and Behavioral Function and Resting-State Functional Connectivity in Patients with Dementia: A Pilot Trial
Institutes – University of California San Francisco & the Veterans Affairs USA
[ Published Study Device: Vielight Neuro Gamma ]
Significant Improvement in Cognition in Mild to Moderately Severe Dementia Cases Treated with Transcranial Plus Intranasal Photobiomodulation: Case Series Report
Co-authoring institutes – Harvard Medical School, Boston University School of Medicine
[ Published Study | Device: Vielight Neuro Gamma ]
Vielight Neuro RX Gamma – Alzheimer’s Disease Pivotal Phase III Clinical Trial (n=228)
Co-institutes – St Michael’s Hospital, Toronto
[ Clinical Trial Information Link ] (228 participants | 3 years | Ongoing)
Neuro RX Gamma for Amnestic Mild Cognitive Impairment (aMCI) / Dementia
Co-institutes – Unity Health Toronto, Toronto
[ Clinical Trial Information Link | Device: Vielight Neuro Gamma ]
Revitalizing Cognition in Older Adults at Risk for Alzheimer’s Disease With Near-Infrared Photobiomodulation
Institutes – University of Florida, University of Arizona, National Institute on Aging (NIA)
[ Clinical Trial Information Link | Device: Vielight Neuro Gamma ] (168 participants | 5 years | Registration)
Impact of Photobiomodulation (PBM) on Biomarkers of Alzheimer’s Disease
Institutes – University of California San Francisco UCSF
[ Clinical Trial Information Link / Device: Vielight Neuro Gamma ] (16 participants | 1.5 years | Ongoing)
- Traumatic Brain Injury / Concussion
Transcranial Photobiomodulation Treatment Effects In Former Athletes With Repetitive Head Hits (n=49)
Institutes – Department of Neurology, University of Utah
[ Published Abstract Link | Device: Vielight Neuro Gamma ] (49 participants | Pending publication)
Transcranial Photobiomodulation Treatment: Significant Improvements in Four Ex-Football Players with Possible Chronic Traumatic Encephalopathy
Institutes – Boston University School of Medicine, Harvard Medical School
[ Published Study Link | Device: Vielight Neuro Gamma ]
Changes in Brain Function and Structure After Self-Administered Home Photobiomodulation Treatment in a Concussion Case
Institutes – VA Advanced Imaging Research Center, San Francisco VA Health Care System, Departments of Radiology & Biomedical Imaging and Psychiatry & Behavioral Sciences, University of California, San Francisco
[ Published Study Link | Device: Vielight Neuro Gamma ]
Photobiomodulation to Improve Cognition in TBI, With fMRI
Institutes – Boston Veterans Affairs Research Institute
[ Clinical Trial Information Link | Device: Vielight Neuro Gamma ] (20 participants | 1 years | Ongoing)
- Parkinson’s Disease
Improvements in clinical signs of Parkinson’s disease using photobiomodulation: A prospective proof-of-concept study
Institutes – University of Sydney, University of New South Wales, Griffith University
[ Published Study Link Device: Vielight Neuro Gamma ]
Pilot Intervention With Near Infrared Stimulation
Institutes – University of Florida
[ Clinical Trial Information Link | Device: Vielight Neuro Gamma ] (135 participants | 4 years | Ongoing)
Evaluation of dose of Photobiomodulation (Light) Therapy and Physiotherapy for Improving Quality of Life Outcomes and Mobility in Parkinson’s Disease (Pilot)
Institutes – University of Sydney, University of Brisbane
[ Clinical Trial Information Link | Device: Vielight Neuro Gamma ] (16 participants | 1.5 years | Ongoing)
- PTSD and Gulf War Illness
Improvements in Gulf War Illness Symptoms After Near-Infrared Transcranial and Intranasal Photobiomodulation: Two Case Reports
Institutes – University of California San Francisco & the Veterans Affairs USA
[ Published Study Link | Device: Vielight Neuro Alpha ]
- Autism Spectrum Disorder (ASD)
Transcranial Photobiomodulation for the Treatment of Children with Autism Spectrum Disorder (ASD): A Retrospective Study
Institutes – Neurodevelopment Division, Istituto di Neuroscienze, Italy, Department of Psychiatry, Albert Einstein College of Medicine, NY
[ Published Study Link Device: Vielight Neuro Duo ]
- Covid-19
Vielight RX Plus for the Treatment of COVID-19 Respiratory Symptoms
Various clinical sites
[ Pre-print of Results ] (295 participants | 1 year | Pre-publication)
- Post-Covid
A Pilot Study Evaluating the Efficacy of the Vielight Neuro RX Gamma in the Treatment of Post COVID-19 Cognitive Impairment
Various clinical sites
[ Clinical Trial Information ] (36 participants | 1 year | Recruitment)
- Cognitive Enhancement and EEG-Neuromodulation
Enhancement of Divergent Creative Thinking After Transcranial Near-Infrared Photobiomodulation Over the Default Mode Network
Institutes – University of Deusto | University of Montpellier | Pennsylvania State University
[ Published Study Link Device: Vielight Neuro Gamma ]
Pulsed Near Infrared Transcranial and Intranasal Photobiomodulation Significantly Modulates Neural Oscillations: a pilot exploratory study
Institutes – Centre for Therapeutic Brain Intervention, Centre for Addiction and Mental Health, Toronto, Ontario, Canada
[ Published Study Link (Nature, Scientific Reports, 2019) | Device: Vielight Neuro Gamma ]
Modulation of cortical oscillations using 10hz near-infrared transcranial and intranasal photobiomodulation: a randomized sham-controlled crossover study
[ Abstract Text Link | Device: Vielight Neuro Alpha ]
- Bioenergetics and Cellular Enhancement
Near-Infrared Photobiomodulation of Living Cells, Tubulin, and Microtubules In Vitro
Institutes – Department of Physics and the Department of Chemistry, University of Alberta | Scholes Lab, Department of Chemistry, Princeton University, Princeton, NJ, United States | Department of Mechanical and Aerospace Engineering, Politecnico di Torino, Turin, Italy | Vielight Inc
[ Published Study Link | Device: Vielight Neuro Alpha ]
Raman Spectroscopy Reveals Photobiomodulation-Induced α-Helix to β-Sheet Transition in Tubulins: Potential Implications for Alzheimer’s and Other Neurodegenerative Diseases
Institutes – University of Alberta, Polytechnic University of Turin, Freie Universit¨at Berlin, Silesian University of Technology
[ Pre-print | Device: Vielight Neuro Alpha ]
- Systemic, Anti-Viral and Anti-Microbial
Vielight RX Plus for the Treatment of COVID-19 Respiratory Symptoms
Various clinical sites
[ Pre-print of Results ] (295 participants | 1 year | Pre-publication)
Antimicrobial Photodynamic Inactivation Mediated by Tetracyclines in Vitro and in Vivo: Photochemical Mechanisms and Potentiation by Potassium Iodide
Institutes – Harvard Medical School
[ Published Study Link | Vielight MIP ]
Antimicrobial Blue Light Therapy for Infectious Keratitis: Ex Vivo and In Vivo Studies
Institutes – Harvard Medical School
[ Published Study Link | Vielight MIP ]
Antimicrobial blue light inactivation of Candida albicans: In vitro and in vivo studies
Institutes – Harvard Medical School
[ Published Study Link | Vielight MIP ]
Whats Included
Additional information
- Near-infrared light is nearly invisible to the naked eye, making the beam appear dimmer than its power density.
- Each device comes with:
1) 1 Neuro headset
2) 1 Neuro intranasal applicator
3) 1 Charger
4) 1 Device pouch
5) 1 Silicon sleeve
6) 1 Carry case
FAQS
What wavelength does the Vielight Neuro emit?
The Vielight Neuro emits pulsed 810nm NIR energy to enable brain stimulation through the photobiomodulation process.
Why the 810nm wavelength?
The 810nm wavelength has the deepest penetration through tissue and bone, enabling photonic transmission to neuronal mitochondria.
What is the difference between the Duo, Gamma and Alpha models?
The Gamma model pulses NIR light energy at 40 Hz, which has a neuromodulating effect on Gamma neural oscillations.
Effects are an increase of focus, information processing, memory improvement, energizing.
The Alpha model pulses NIR light energy at 10 Hz. This frequency is similar to neural alpha waves. Theoretical brain stimulation effects on alpha waves have a correlation with the brain’s resting state, offering support for mindfulness, learning, relaxation, sleep improvement.
The Duo makes both of these pulse rates available to you – having both pulse rates brings the most benefits.
How much power do your brain photobiomodulation devices generate?
Our brain photobiomodulation devices generate sufficient power to penetrate the skull and produce statistically significant results in independent research studies.
What is the recommended usage?
The general recommended usage is to use once every other day before experimenting with more frequent usage, not exceeding once per day. For more serious cognitive impairment, the recommendation is once per day, six days a week. Please note these recommendations are theoretical and anecdotal.
SHIPPING AND RETURN POLICY
How do you ship?
We ship globally to most countries. It takes 5 to 7 business days for your purchase to reach its destination, once your order has been processed.
We ship via local postal services within Canada, Australia, and the United States, and internationally using private courier. Vielight does not charge taxes, but your local country’s customs agencies will reach out for any import taxes that they may be required to pay, and we are unable to waive any of these fees. The average shipping time for international orders may be longer depending on inspections by your local customs agency.
What are the shipping requirements for each country?
USA: Deliveries of all Neuro, XPlus, Vagus, and other products with a power bank require an Adult Restricted Delivery Signature, where only the receiver can sign for delivery and proof of ID is required.
Canada: Deliveries to all Canadian addresses require presentation of an ID card at pick-up.
Australia: Deliveries to all Australian addresses require a delivery signature.
Outside of Australia, Canada, and the USA: Delivery Signature and payment of customs duty are required.
What is your return and refund policy?
We offer an 80% refund within 6 months of purchase if you’re not satisfied with your Vielight device.
This applies only to:
-
Direct purchases from Vielight, or
-
Purchases from authorized global resellers
To request a refund, please contact the point of purchase directly.
🔒 Note: Intranasal applicators purchased separately are non-refundable.
Downloads
You may also like
Contact form
Got a question or need assistance? We're here for you!
Thanks for visiting our website. We value your time and are always happy to help. Please fill out the contact form below with your name, email address, and message, and we'll get back to you as soon as possible.
We look forward to hearing from you and assisting with any questions or concerns you may have.








